Vaccine resources
The Common Health Coalition is bringing together payers, providers, health systems, and public health leaders to prepare for and respond to the 2025-2026 respiratory virus season and to provide situational awareness around changes to childhood immunizations. Our goal: a coordinated response that improves access to vaccines for patients and communities. All resources below are free to use and indicate the date they were last updated. More will be added as the situation evolves.

Evidence
The Vaccine Integrity Project (VIP) is an independent group of vaccine experts formed in response to federal vaccine policy changes. They met on August 19, 2025, to review the latest evidence on COVID-19, flu, and RSV vaccine effectiveness and safety. The executive summary of that meeting can be found here, and a recording can be found here.
VIP recently published an evidence review of Hepatitis B vaccination at birth, concluding there is no benefit to delaying a first dose.
Experts at the Evidence Collective, a group of trusted health communicators who unite to deliver clear, evidence-based information, have published helpful explainers on the vaccine evidence, including pre-bunk documents for ACIP meetings and others, all found here.
Recommendations
Medical specialty societies publish evidence-based recommendations for vaccines each year. These recommendations help guide the public’s decision-making, clinical practice, payer coverage decisions, and health system purchasing. If a specialty society has not yet released 2025-2026 guidance, its 2024-2025 recommendations remain applicable until updated.
Childhood Immunizations
Respiratory season (COVID, Flu, RSV)
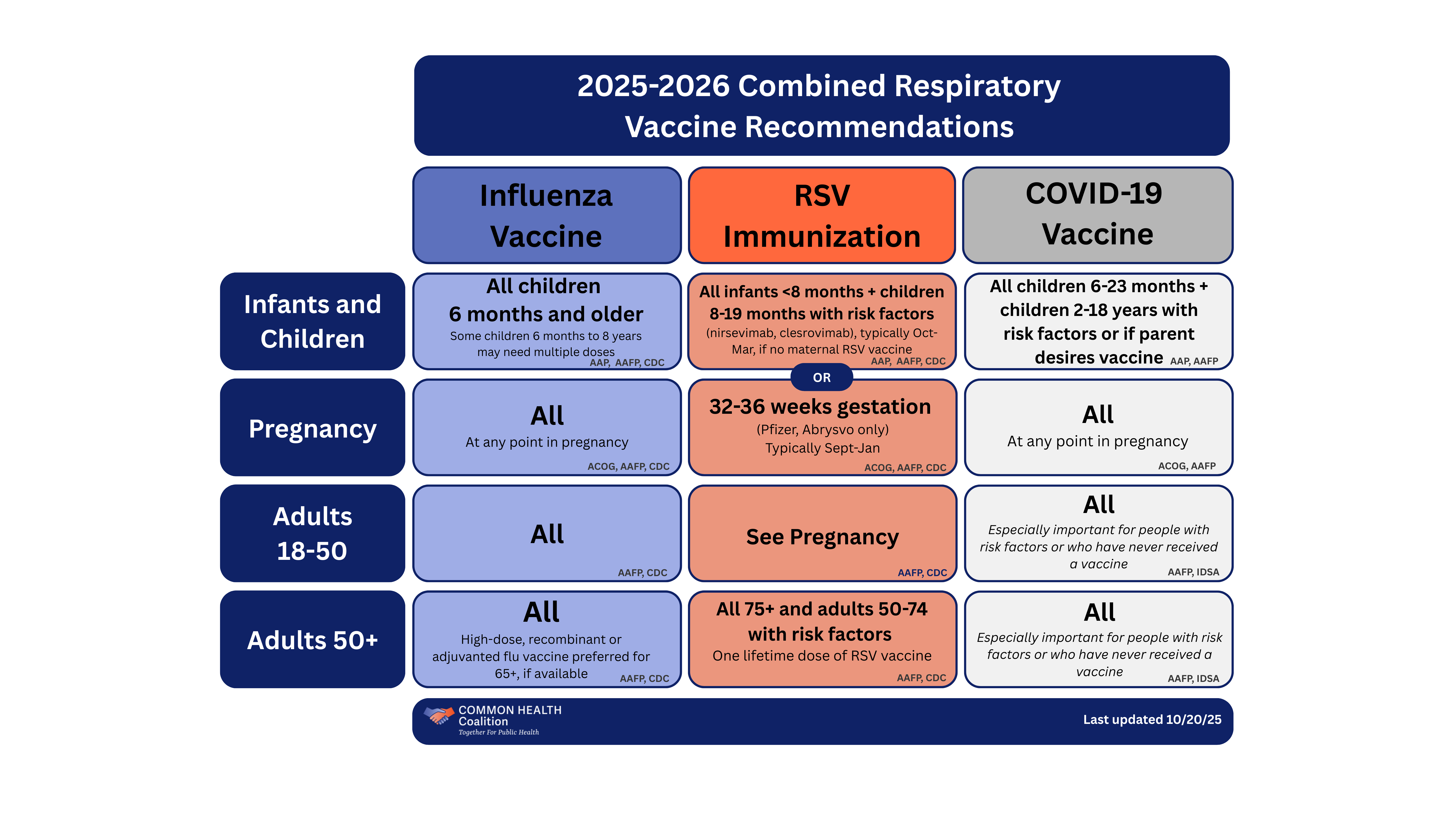
Below is the Coalition’s aggregate summary of updated recommendations from various sources for flu, RSV, and COVID-19 vaccines for infants and children, pregnant people, adults 18-50, and adults 50+ based on the latest guidelines.
See below for more the recommendations from:
Respiratory Immunizations (COVID, Flu, RSV)
 Anticipated impact of changes to childhood immunization schedule [Infographic]
Anticipated impact of changes to childhood immunization schedule [Infographic]
Outlines possible cascading impacts resulting from changes to the childhood immunization schedule across patients, providers, manufacturers, public health, and payers over time and key actions that payer organizations, state leaders, health systems, and providers can take to mitigate these impacts, maintain access, and reinforce trust.
 December 2025 ACIP Meeting Explainer
December 2025 ACIP Meeting Explainer
Frequently asked questions about the Hepatitis B vaccine recommendations from the December 2025 ACIP Meeting.
 COVID-19 Vaccination Access: Fall/Winter 2025 State Actions
COVID-19 Vaccination Access: Fall/Winter 2025 State Actions
Provides an overview of actions to expand COVID-19 vaccine access taken by states thus far.
 States toolkit
States toolkit
Guidance for states to align on vaccine distribution and outreach.
 Providers toolkit
Providers toolkit
Tools to help providers integrate vaccine guidance into practice and patient communications.
 Payers toolkit
Payers toolkit
Resources to guide payer vaccine coverage decisions and communications.
 Adult COVID-19 vaccine dosing quick reference guide
Adult COVID-19 vaccine dosing quick reference guide
1-page quick reference guide on dosing for COVID-19 vaccine products.
 Hot topics on respiratory vaccines: Clearing the air on liability and practice considerations
Hot topics on respiratory vaccines: Clearing the air on liability and practice considerations
This webinar—co-hosted by the Common Health Coalition and the American Academy of Pediatrics (AAP)—brought together legal, clinical, and communication experts to clarify key considerations around shared decision-making and professional protections. Click the link to download the slides or watch the webinar here.
 Shared clinical decision-making guide on vaccines for clinicians
Shared clinical decision-making guide on vaccines for clinicians
Provides HCPs with an overview of shared clinical decision-making related to vaccines. The guide includes examples, frequently asked questions, and other helpful resources.
 2025-2026 COVID-19 vaccine availability, by state
2025-2026 COVID-19 vaccine availability, by state
Provides an overview of COVID-19 vaccine availability and actions for access by state. [Updated regularly]
 2025-2026 combined respiratory vaccine recommendations
2025-2026 combined respiratory vaccine recommendations
Provides an aggregate summary of updated recommendations from various sources for flu, RSV, and COVID-19 vaccines for infants and children, pregnant people, adults 18-50, and adults 50+ based on the latest guidelines.
 Operational timeline for respiratory vaccines
Operational timeline for respiratory vaccines
Summarizes the prior years’ process to operationalize this season’s flu, RSV, and COVID-19 vaccines.
 Respiratory virus coverage analysis
Respiratory virus coverage analysis
Outlines the current coverage landscape for commercial payers, government payers, and certain programs for COVID-19 flu vaccines and RSV immunizations.
 ACIP and CDC vaccine recommendation updates
ACIP and CDC vaccine recommendation updates
Summarizes changes to immunization recommendations across key populations made during the April and June 2025 ACIP meetings and revised guidance from the CDC on COVID-19 vaccines in May 2025.
 Vaccine Injury Compensation Program notes
Vaccine Injury Compensation Program notes
Outlines how changes to ACIP recommendations may impact federal vaccine injury compensation programs for flu and COVID-19 vaccines and RSV immunizations, focusing on coverage, liability protections, and regulatory processes.
 Provider liability, coverage, and scope: Scenario tables
Provider liability, coverage, and scope: Scenario tables
Helps providers, policymakers, and payers understand the implications of policy decisions linked to FDA labeling and ACIP recommendations.
 Paying for Childhood Vaccines
Paying for Childhood Vaccines
Summarizes the Vaccines for Children Program and answers common questions.
 Respiratory season vaccine FAQs
Respiratory season vaccine FAQs
Helps health care and public health leaders navigate uncertainty during the 2025-2026 respiratory virus season.
 High-risk criteria for COVID-19
High-risk criteria for COVID-19
Compares how various health agencies define high-risk criteria for severe COVID-19 illness.
 Coverage and Administration of COVID-19 Vaccine for Pregnant People
Coverage and Administration of COVID-19 Vaccine for Pregnant People
Summarizes current policies to the coverage and administration of the COVID-19 vaccine for pregnant people.
 Shared clinical decision-making recommendation: COVID-19 vaccine for children and youth
Shared clinical decision-making recommendation: COVID-19 vaccine for children and youth
Summarizes how the CDC recommendation for “shared clinical decision-making” for the COVID-19 vaccine for children and youth affects coverage and administration of the vaccine (issued May 2025).
 Coverage and administration of flu vaccines with thimerosal
Coverage and administration of flu vaccines with thimerosal
Summarizes how flu vaccine coverage and administration will be largely unaffected by ACIP’s recommendation to prohibit thimerosal in vaccines.
 Vaccine Education Center
Vaccine Education Center
The Children’s Hospital of Philadelphia provides up-to-date and reliable information about vaccines across the lifespan.
 Adult vaccinations tip sheet
Adult vaccinations tip sheet
Tip sheet for providers on U.S. adult vaccines and tools to help adults catch up on needed vaccinations.
 Public Health Communications Collaborative
Public Health Communications Collaborative
Talking points, resources for your community, and tools to help you communicate about vaccine development, safety, and effectiveness. Featured resources include communicating about flu and COVID-19, vaccine mandates, and messaging guidance for reinforcing that vaccines do not cause autism.
 VaccineResourceHub.org
VaccineResourceHub.org
The nation’s largest hub for vaccine communications designed for community-based organizations, with content in over 50 languages.
 Immunize.org
Immunize.org
Hundreds of free immunization education materials for health care providers and patients.
 Updates to the COVID-19 vaccine guidelines
Updates to the COVID-19 vaccine guidelines
Q&A from Your Local Epidemiologist about the recent changes to the COVID-19 vaccine guidelines.
 Infodemiology.com
Infodemiology.com
Insights into trending vaccine narratives designed for health care providers and public health professionals.
 The Evidence Collective
The Evidence Collective
Focused, actionable briefs that synthesize critical vaccine information.
 Trusted Messenger Program
Trusted Messenger Program
The Trusted Messenger Program (TMP) empowers health care providers, health systems, and integrated delivery networks to drive quality outcomes through the practice of evidence-based communication.
 Mitigating Misinformation
Mitigating Misinformation
One-page summary on how to effectively communicate with skeptical-but-persuadable patients.
 Messaging for trust
Messaging for trust
Trust messaging tools from the Coalition for Trust in Health & Science to assist members in communicating effectively about health and science.
 Vaccine development, safety, and effectiveness
Vaccine development, safety, and effectiveness
Talking points, resources for your community, and tools to help you communicate about vaccine development, safety, and effectiveness.